Michael Renard, executive vice president of bioprinting company Organovo, explains how 3D printing could one day be used to produce replacement tissue, vessels and organs in this interview conducted for our print-on-demand magazine Print Shift (+ transcript).
In the interview Renard describes how Organovo is applying 3D printing to cell biology and tissue engineering.
"We’re working with small pieces of tissue at the moment - a small piece of blood vessel or liver," he says. "Once you have the cells ready, we can print something in a few hours."
He also discusses how the technology can be used for experimental drug testing: "Being able to provide functional living human tissues will provide drug-discovery scientists with entirely new means to test drug candidates."
Although supplemental tissues such as patches to assist heart conditions may reach the clinic soon, he thinks that use of "more advanced replacement tissues will most likely be in 20 years or more."
The interview forms part of a feature on the way 3D printing is transforming the healthcare industry in Print Shift, our one-off, print-on-demand magazine about this emerging technology.
The magazine was created by the Dezeen editorial team and produced with print-on-demand publisher Blurb. For more information about Print Shift and to see additional content, visit www.dezeen.com/printshift.
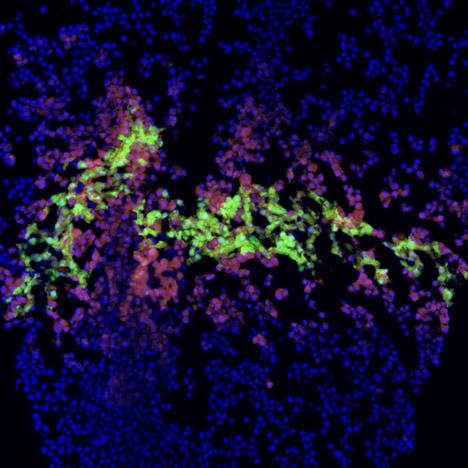
Top image: cross-section of bioprinted human liver tissue.
Here's an edited transcript of the interview, conducted by Claire Barrett:
Claire Barrett: Tell me how Organovo’s 3D printing research began?
Michael Renard: The concept for printing tissues came out of Professor Gabor Forgac’s research at the University of Missouri, enabled through a $5 million grant from the National Science Foundation. That work was about using living cells and depositing cells in an architecture that could create tissue.
It led to the creation of Organovo as a company, which acquired that intellectual property exclusively. Gabor worked mostly with non-human cell sources to build structures, layer by layer. Through that science we arrived where we are today.
Claire Barrett: Is it possible to print an organ?
Michael Renard: Bioprinting should be thought of as the first step in building fully functional tissue. The printing starts a process to create a continuous piece of tissue. That early tissue construct is moved to a bioreactor where it grows and differentiates into its final form. We’re the only company doing it. Our approach is consistent with other forms 3D printing because it’s an additive process, but what is unique to Organovo is our application of the process in the field of cell biology and tissue engineering.
Claire Barrett: How does it work?
Michael Renard: Tissues are built layer by layer, using a combination of hydrogel and cell aggregates deposited in specific spatial arrangements that are programmed into the bioprinter. A wide variety of shapes and orientations can be created using the combination of these materials.
When you deposit cells they have to be the right cells and in the right biological state; the hydrogel holds them in the right place. Then the cells fuse, form junctions, and the hydrogel can be removed to yield a tangible piece of material made up entirely of human cells.
Claire Barrett: How long does it take?
Michael Renard: It all depends what you’re trying to grow. We’re working with small pieces of tissue at the moment - a small piece of blood vessel or liver, for example, so our time from printing to maturity of the tissue can be quite quick. Once you have the cells ready, we can print something in a few hours. It will then take a few days for it to fuse and become anatomically correct, and begin to exhibit expected metabolic properties.
It is unknown how long it will take to build larger, organ-sized tissues. We are researching ways to grow a vascular system as part of the tissue design; that is needed to feed tissue grown on a large scale, without which cell death will occur as tissues expand in size.
Claire Barrett: Are certain tissues easier to grow?
Michael Renard: Virtually all tissues have a specific design and repeating patterns. Each tissue has a consistent set of characteristics, such as certain cell types that create capillary systems, nerves and collagens. These patterns and symmetry can help as the scientific advances and discoveries with one tissue will better inform how to approach the creation of subsequent tissues.
Claire Barrett: How is it used in drug discovery and what are the benefits?
Michael Renard: Being able to provide functional living human tissues will provide drug-discovery scientists with entirely new means to test drug candidates and study their effects in an environment most like that of the drug administered in the human body. This can both improve the safety of potential drugs and help determine whether a drug should be taken forward in very expensive human clinical trials. The end result can be a significant improvement in the efficiency of safety and efficacy testing.
Further to that, diseased tissue models can be built, giving the scientist a completely new approach for understanding disease and disease progression, with the opportunity to find new targets for building drugs with new mechanisms of action.
Claire Barrett: Is the public worried about the ethics of growing organs in a lab?
Michael Renard: People with chronic or degenerative conditions often live with the constant need for medical and assisted-living care. We can keep people alive, but at a cost to the healthcare system and at a reduced quality of life for the patient. What if we could reverse that process, or replace an organ? That’s what the focus is. There is public interest. People are waiting for transplants, but transplant surgeons lack the tissues to help all those in need. Eighteen people die every day in the US waiting for a transplant.
Claire Barrett: What about tissue rejection? Could you take cells from a person in future and grow tissue for transplant and therefore avoid this issue?
Michael Renard: It has become possible to harvest cells from a person’s own body and use them as a source of therapy. Research over the last decade or so shows that many sources of stem cells can be isolated and these often can be a valuable source of potential therapy from the patient themselves. In concept, a tissue engineered from a person’s own DNA should yield a match, with a much-reduced chance of rejection.
Claire Barrett: How far away are you from creating tissue that can be used in operations?
Michael Renard: In the next 10 years it is possible that supplemental tissues, ones that aid in regeneration, will progress through design, clinical and regulatory testing, making it to the clinic as therapies. Examples may include nerve grafts, patches to assist a heart condition, blood vessel segments, or cartilage for a degenerating joint. But more advanced replacement tissues will most likely be in 20 years or more.
Claire Barrett: What needs to happen to enable the next stage of innovation to take place?
Michael Renard: Supplemental tissues need to be shown to be safe, clinically effective and cost-effective in terms of reducing the total cost of care. Also, the ability to grow larger tissues - solving the challenge of creating a vascular and capillary network as an inherent part of the engineering solution - is the critical next step to advance the science.